Chemistry for Class 10 Students
Chemical Reactions and Equation
In this article, you will be able to know and learn about some important terminologies that are frequently asked while learning and teaching class 10 chemistry.
Terminologies
Balanced chemical equation
A balanced chemical equation refers to the chemical equation in which the number of atoms of each element is the same on both sides of the arrow. For example,3Fe + 4H2O —> Fe3O4 + 4H2
Chemical reaction
Chemical reaction refers to the process in which one or more substances (reactants) are converted to one or more different substances (products).
Combination reaction
A combination reaction refers to a reaction in which a single product is formed from two or more reactants.
Corrosion
Corrosion refers to the gradual degradation or destruction of materials (metals) by any chemical or electrochemical reaction with their environment.
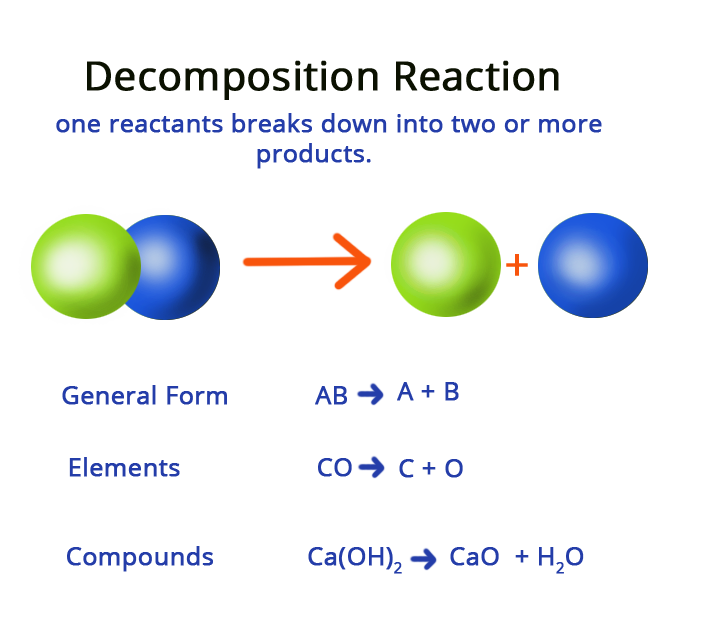
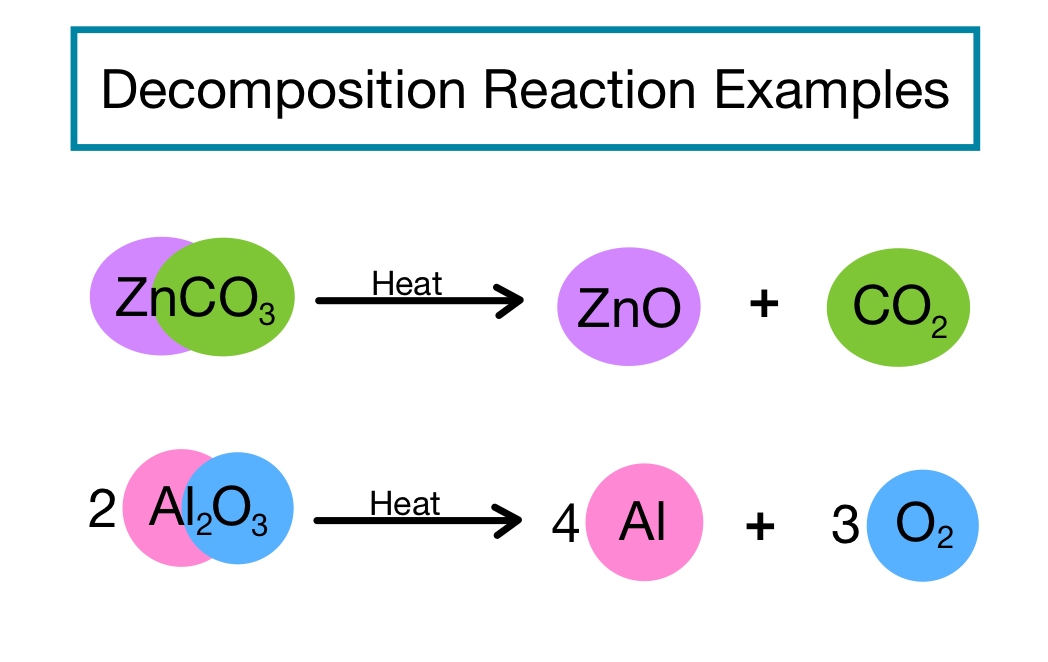
Decomposition reaction
· Decomposition reaction is also known as chemical decomposition or chemical breakdown.
· Decomposition reaction refers to the process or effect of simplifying a single chemical entity into two or more fragments.
Double displacement reaction
· Usually a double displacement reaction results in precipitation.
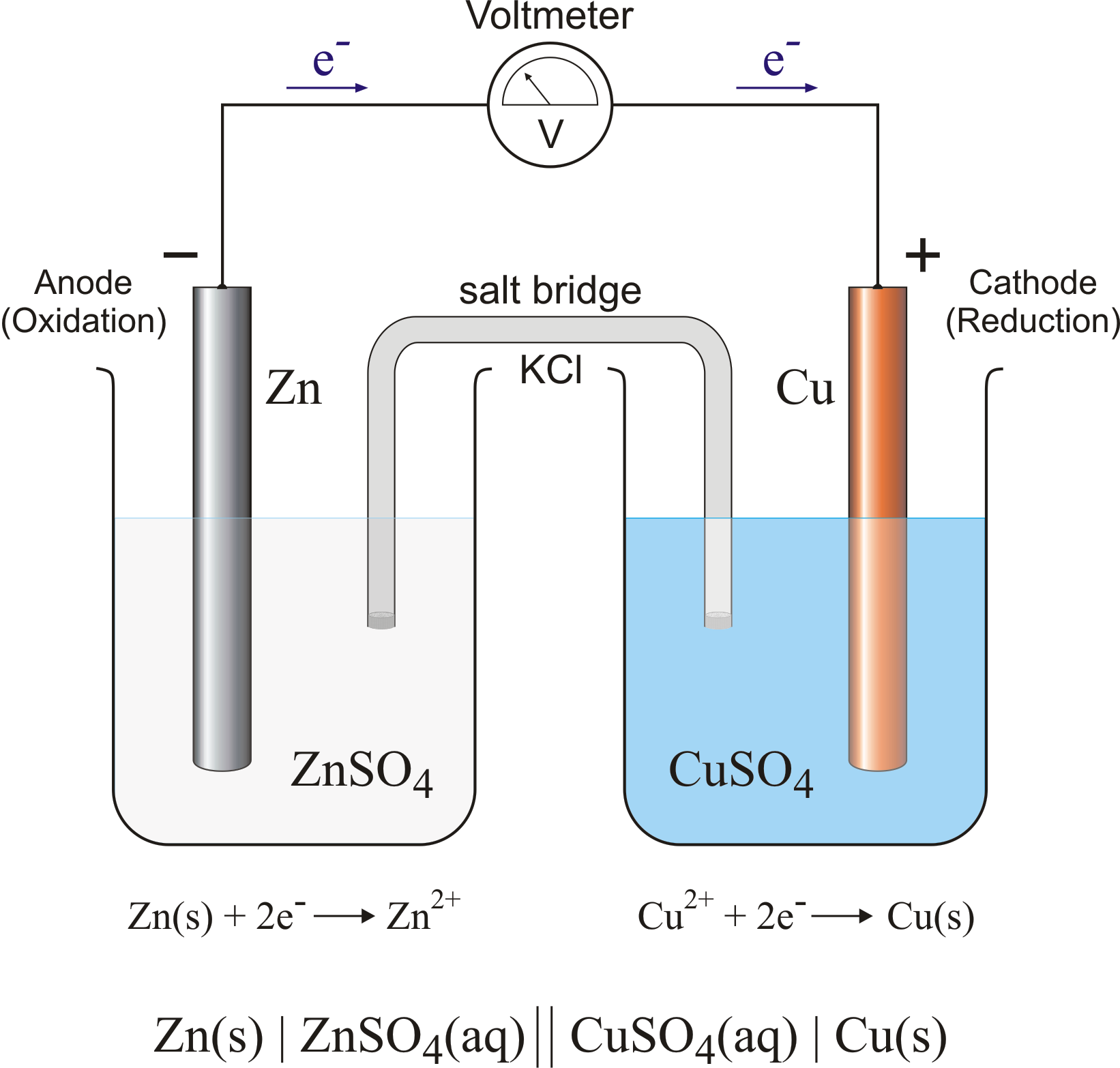
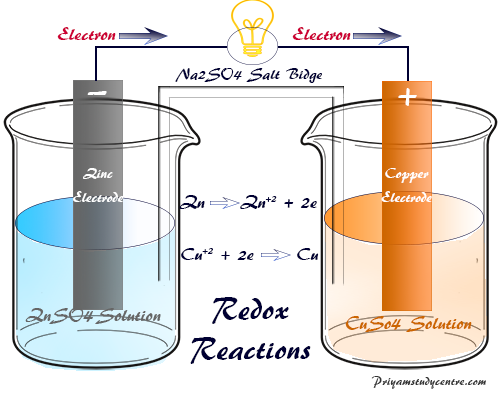
Electrochemical reaction
· Electrochemical reaction refers to the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the electrode, and an ionic conductor.
· Electrochemical reaction takes place with the absorption of electrical energy.
· Some suitable examples of electrochemical reactions are Galvanic cells and Acidulated water that breaks into Hydrogen and Oxygen.
Endothermic reaction
Endothermic reaction refers to the reaction in which energy is absorbed. For example,
2AgBr —> 2Ag + Br2
Exothermic reaction
Exothermic reaction refers to reactions in which heat is released along with the formation of products. For example,
CH4 +2O2 → CO2 + 2H2O
Oxidation reaction
Oxidation reaction refers to the reaction in which a substance loses electrons itoget oxidized.
Precipitate
Precipitate refers to a substance separated from a solution or suspension by chemical or physical change usually as an insoluble amorphous or crystalline solid.
Precipitation reaction
Precipitation reaction refers to the reaction that produces a precipitate.
Product
· Products refer to the substances formed during a chemical reaction.
· Products are substances to the right of the arrow in a chemical equation.
Reactant
· Reactant refers to a substance that takes part in and undergoes change during a reaction.
· Reactants refer to substances to the left of the arrow in a chemical equation.
Redox reaction
Redox reaction refers to a reaction in which both oxidation and reduction take place simultaneously.
Reduction reaction
Reduction reaction refers to the reaction in which a substance gains electrons to get reduced.
Single displacement reaction
A single displacement reaction refers to a chemical reaction in which one element is replaced by another in a compound. For example,
Fe + CuSO4 → FeSO4 + Cu
Thermal decomposition
Thermal decomposition refers to the decomposition reaction which is carried out by heating. For example,
CaCO3 + Heat —-> CaO +O2
To know more visit https://zueducator.blogspot.com












No comments:
Post a Comment